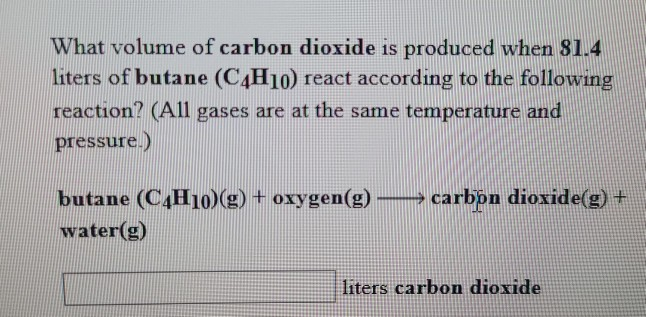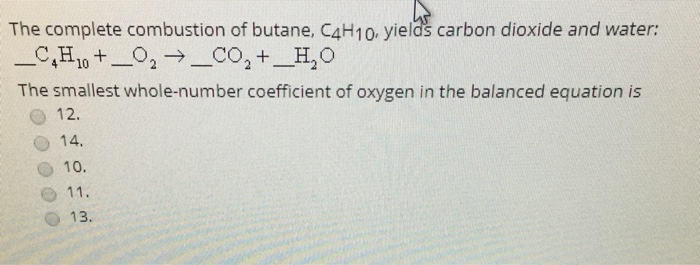When butane burns in oxygen, it produces carbon dioxide and water. This reaction is represented in - Brainly.com
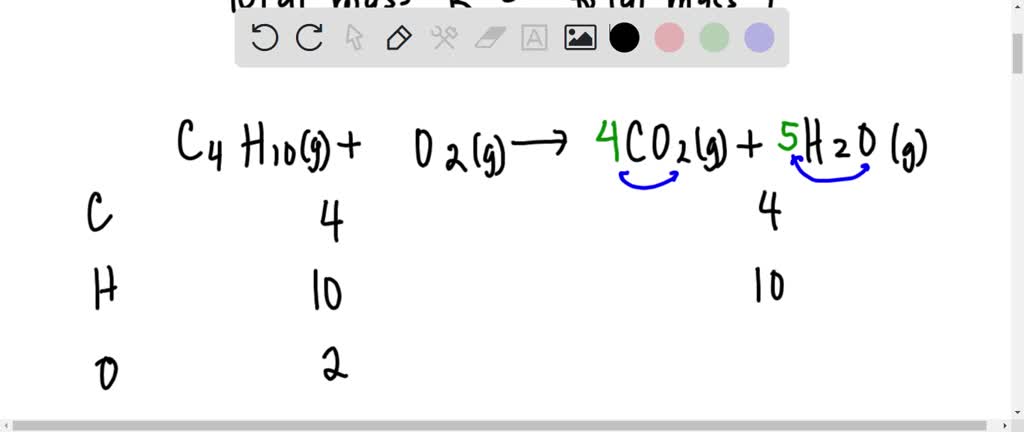
SOLVED: Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseouscarbon dioxide and water vapor.
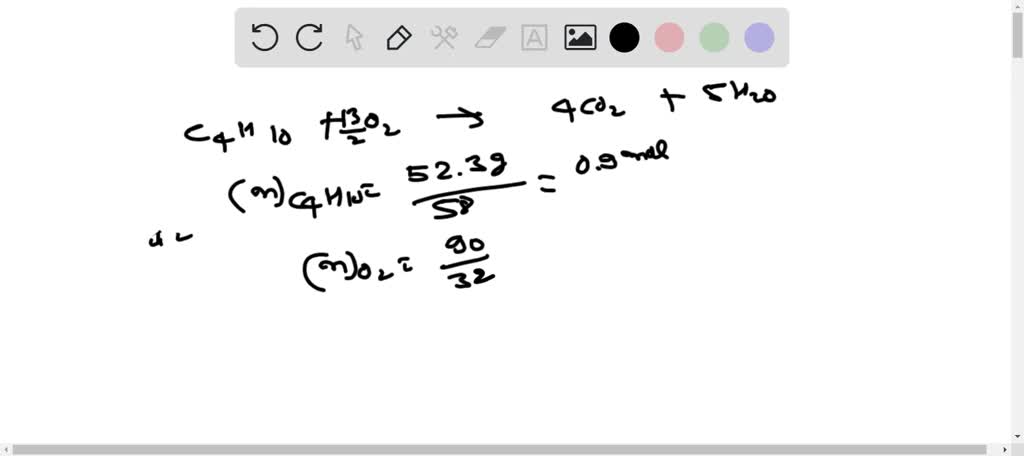
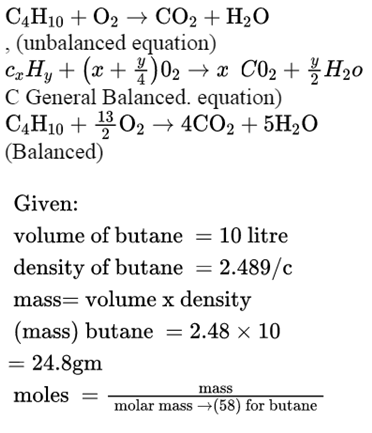
SOLVED: Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 5.23 g of butane is mixed with 9.5 g of oxygen. Calculate the maximum
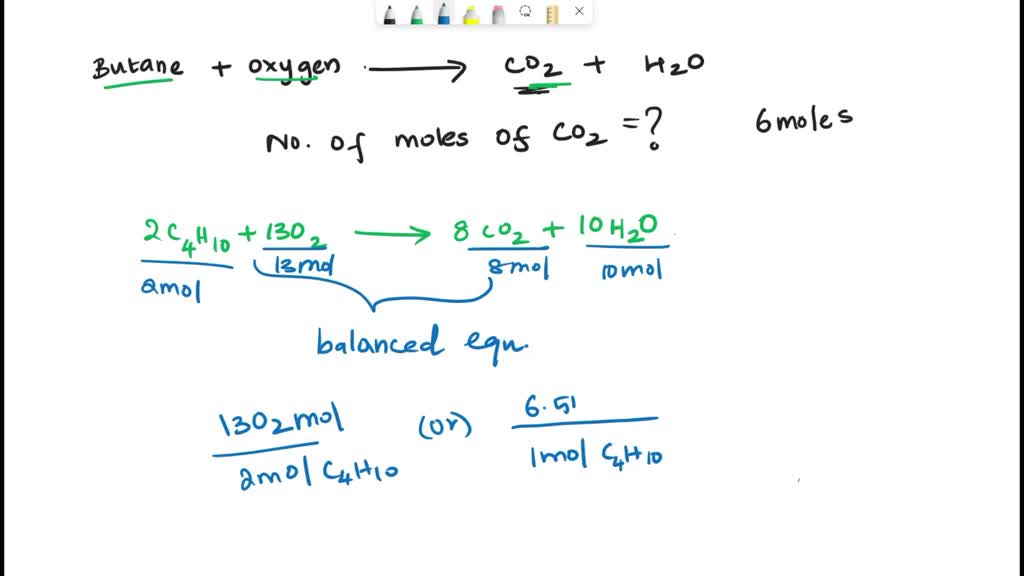
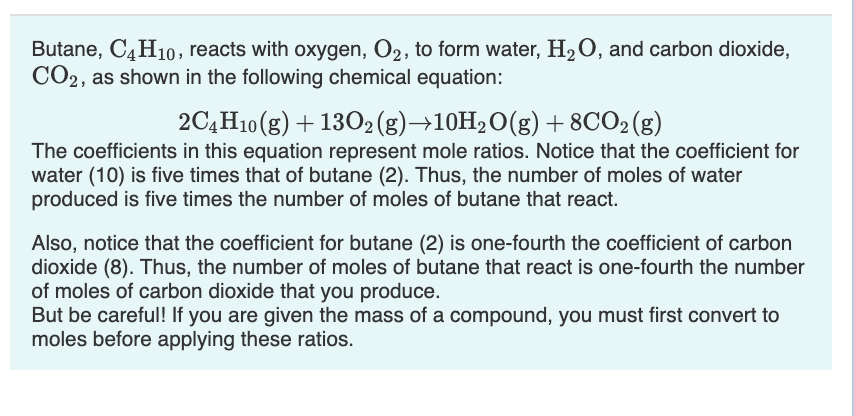
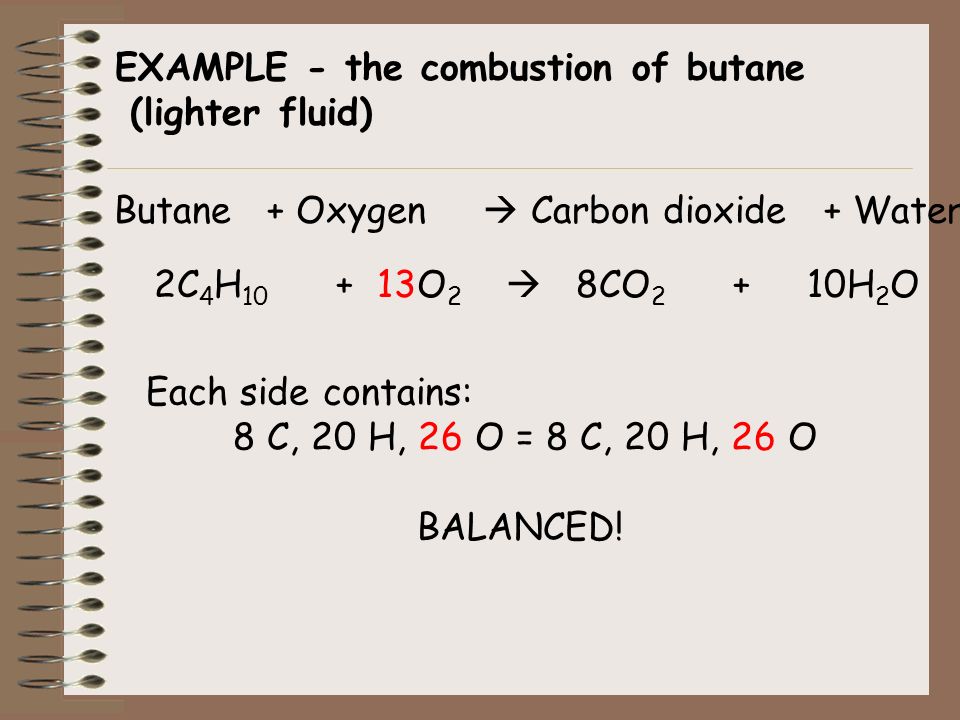
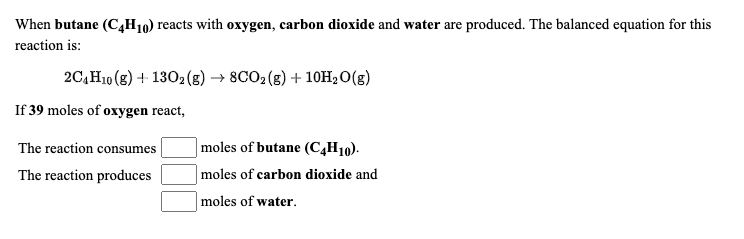
SOLVED: When butane (C4H10) reacts with oxygen, carbon dioxide and water are produced. The balanced equation for this reaction is: 2C4H10 (g) + 13O2 (g) → 8CO2 (g) + 10H2O (g) How

When 0.340 mol of butane, C_4H_{10}, are burned with excess oxygen giving CO_2 and H_2O, how many moles of oxygen are consumed? | Homework.Study.com

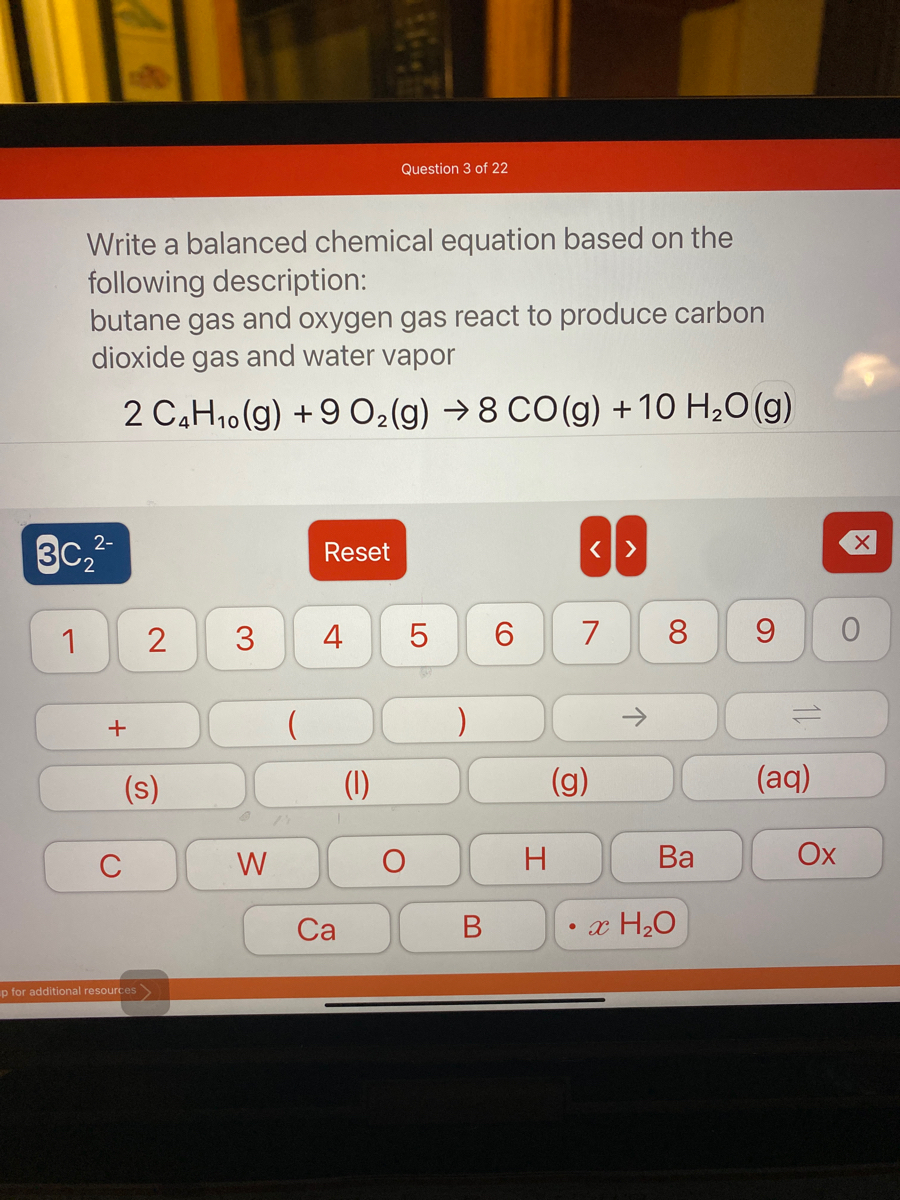
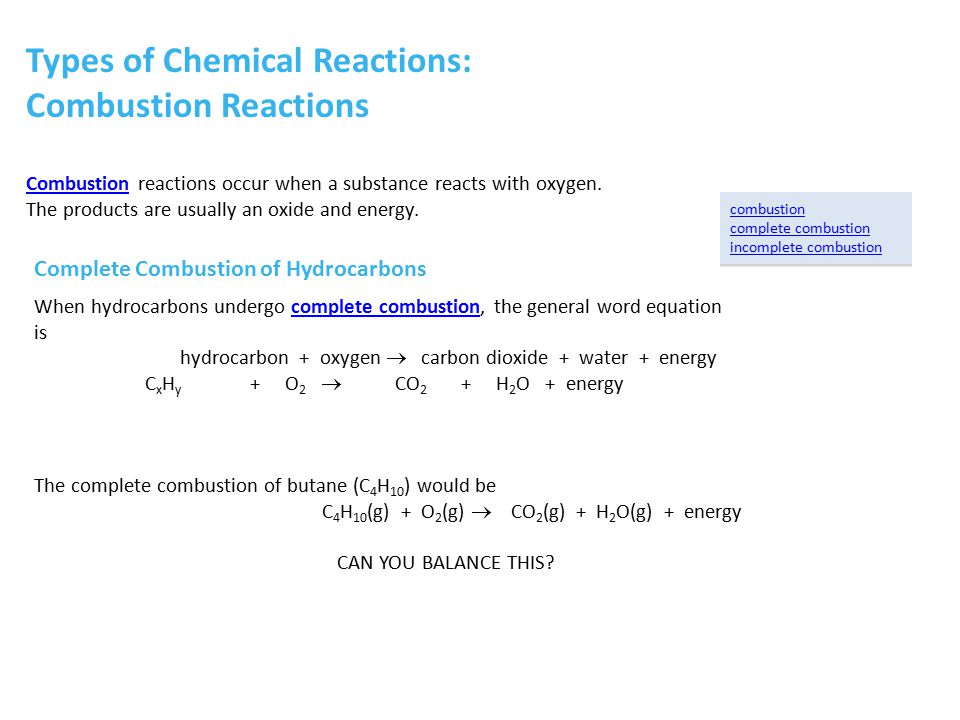
SOLVED: Now balance the chemical reaction by providing the correct whole number coefficients: butane (C4H1o) + oxygen gas yields carbon dioxide water ? JC4H1o JOz 7 Jcoz + HzO Enter