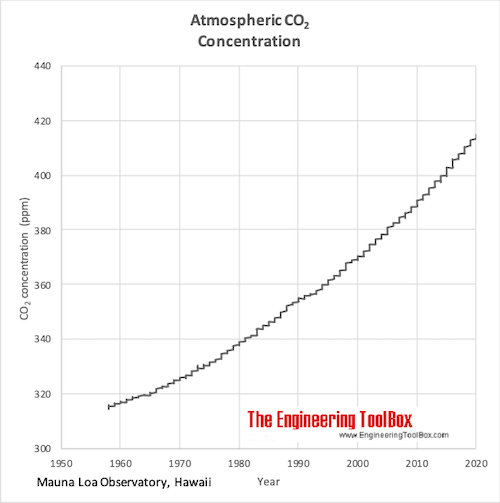
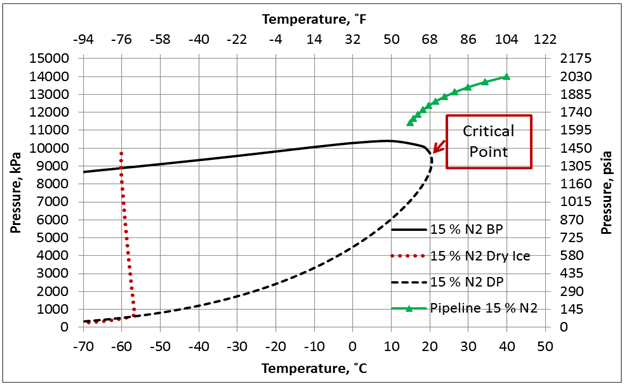
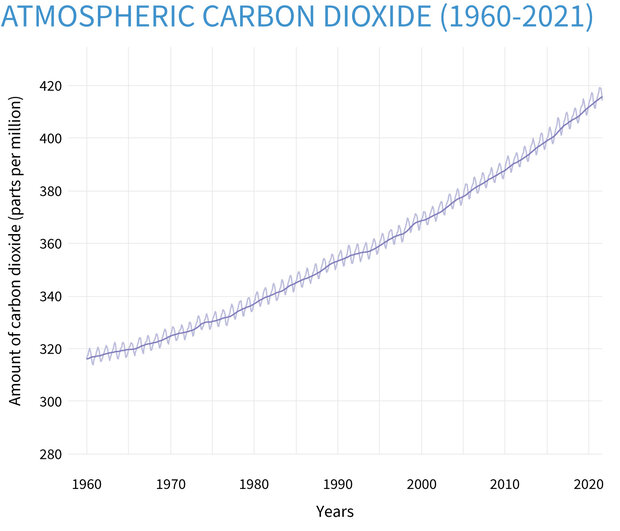
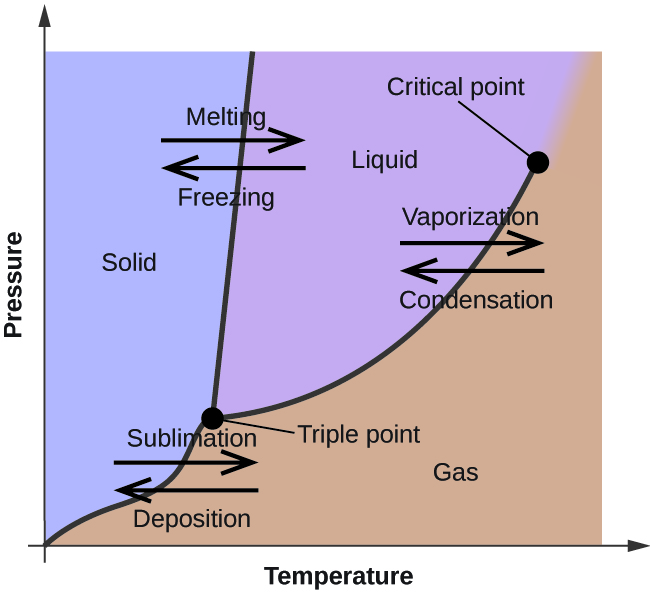
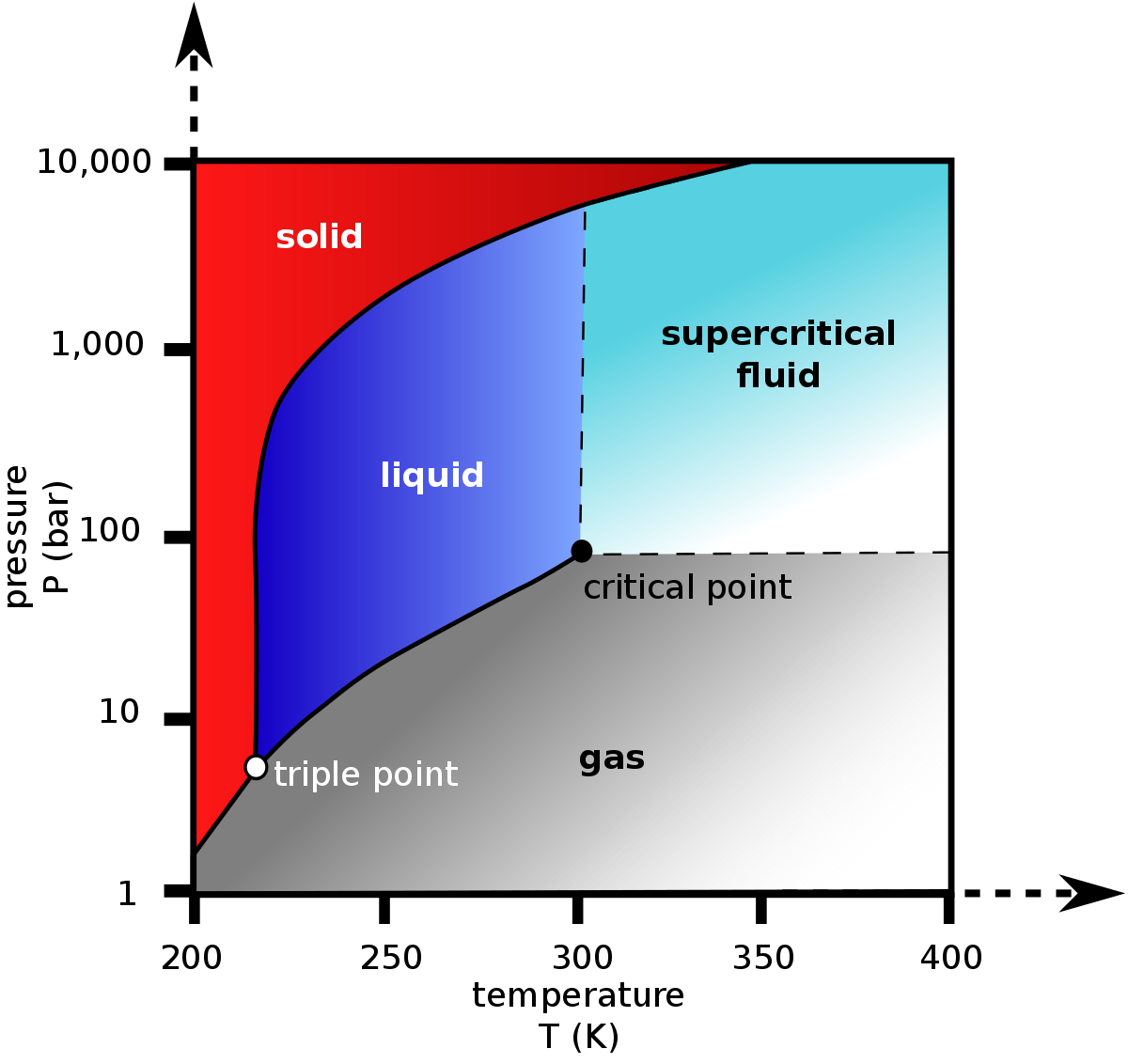
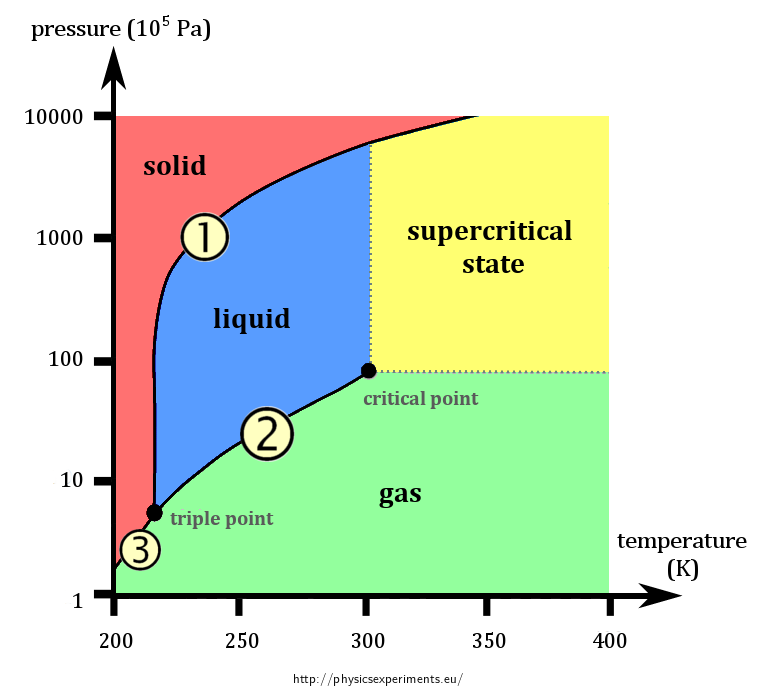
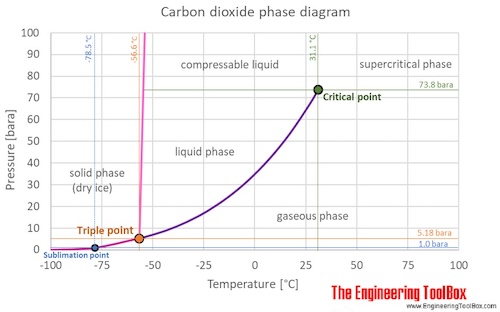
SOLVED: Carbon Dioxide Water Freezing Point (K) Boiling Point Sublimation Point N/A N/A 273 373 195 N/A Liquid Specific Heat Capacity (J/kg-K) Solid Specific Heat Capacity (J/kg K) Latent Heat of Fusion (
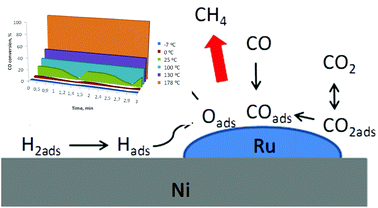
Ultra-low temperature carbon (di)oxide hydrogenation catalyzed by hybrid ruthenium–nickel nanocatalysts: towards sustainable methane production - Green Chemistry (RSC Publishing)

Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point - ScienceDirect