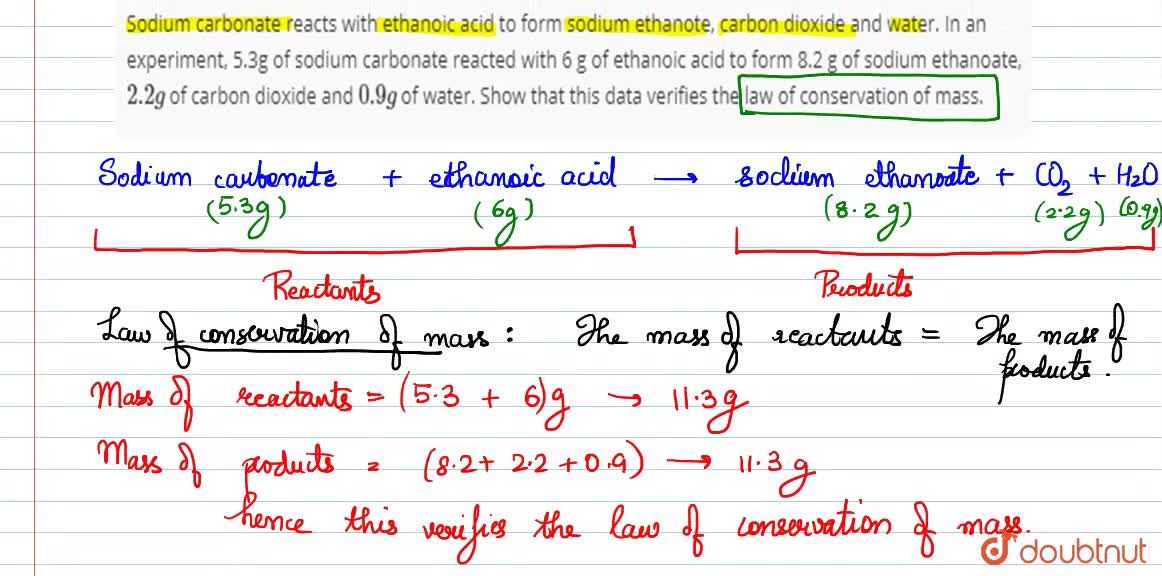
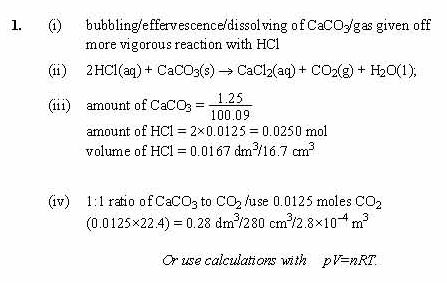
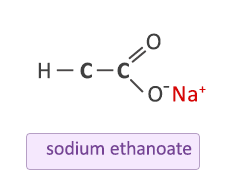
Sodium carbonate reacts with ethanoic acid to form sodium ethanote, carbon dioxide and water. In an experiment, `5.3` g of sodium carbonate reacted with `6` g of ethanoic acid to form `8.2` g of ...
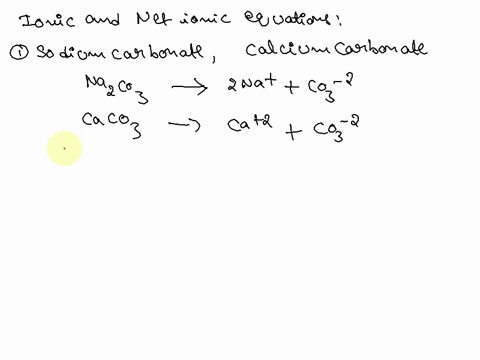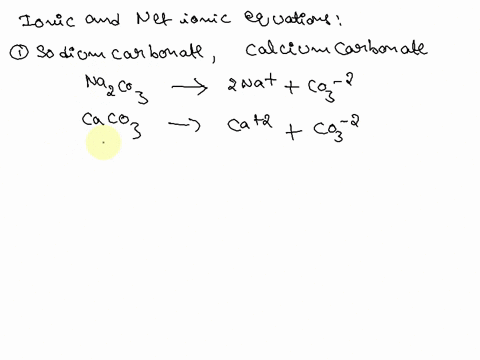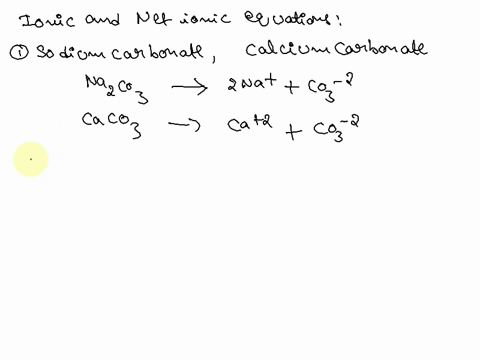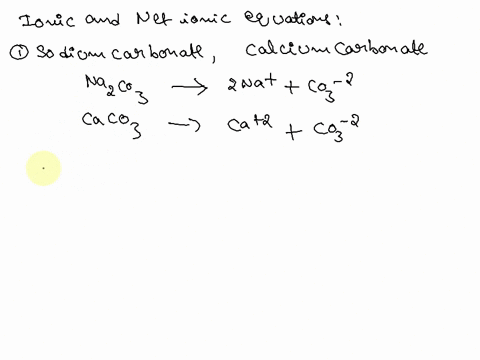
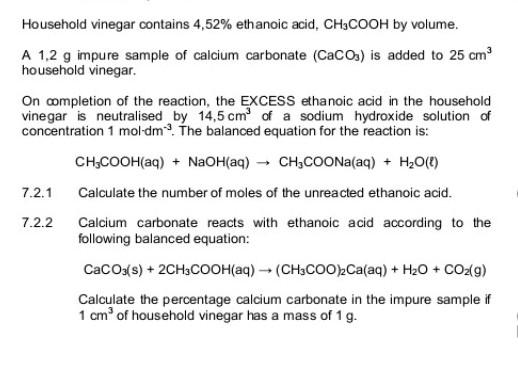
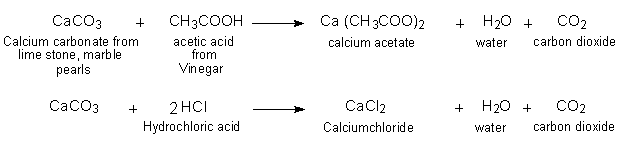
SOLVED: Write the molecular/chemical reaction of calcium carbonate with acetic acid and the net ionic equation.

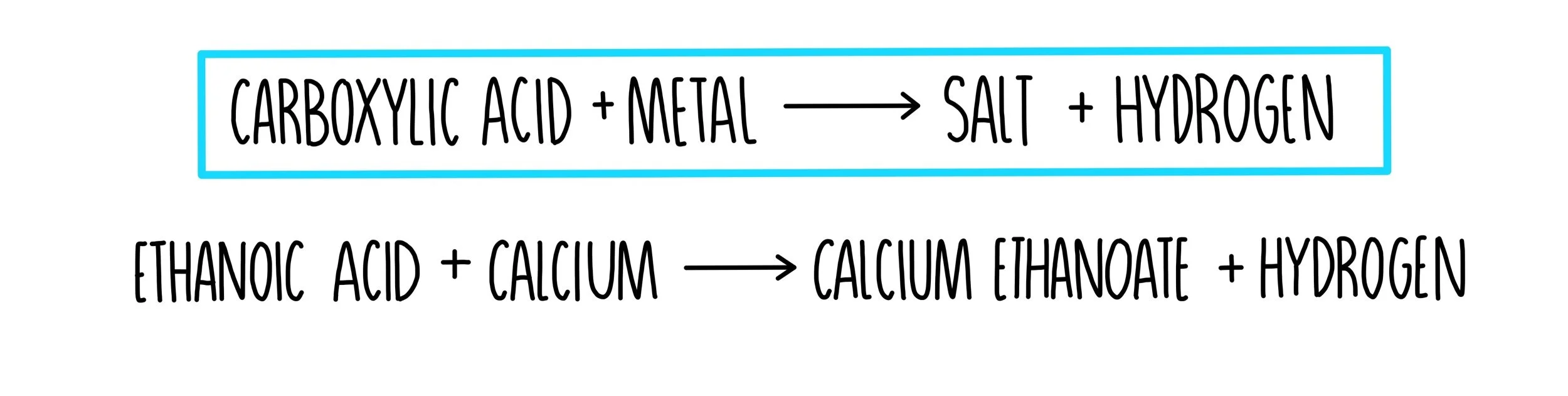
pKa Ka data factors affecting Acidic reactions of carboxylic acids with metals oxides hydroxides carbonates hydrogencarbonate test advanced A level organic chemistry revision notes doc brown


.jpg)



![Carbon] Ethanoic acid - Formation, Properties, Uses [with Reactions] Carbon] Ethanoic acid - Formation, Properties, Uses [with Reactions]](https://d1avenlh0i1xmr.cloudfront.net/fb1b0fbe-d74a-44bb-8d4d-1b41b5aa53a2/reaction-of-ethanoic-acid-with-sodium-carbonate-and-sodium-bicarbonate---teachoo.jpg)


![MCQ] When sodium hydrogen carbonate is added to ethanoic acid a gas MCQ] When sodium hydrogen carbonate is added to ethanoic acid a gas](https://d1avenlh0i1xmr.cloudfront.net/65ccd703-8697-4cd2-9fd1-2b1155ff736e/reaction-of-sodium-hydrogen-carbonate-with-ethanoic-acid---teachoo.png)
.jpg)