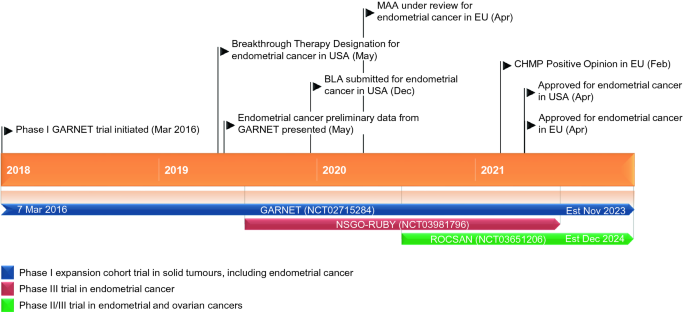
Dostarlimab in Advanced/Recurrent Mismatch Repair Deficient/Microsatellite Instability High or Proficient/Stable Endometrial Can

Phase 3, randomized, open-label study of pembrolizumab plus lenvatinib versus chemotherapy for first-line treatment of advanced or recurrent endometrial cancer: ENGOT-en9/LEAP-001 | International Journal of Gynecologic Cancer

Plain language summary of the GARNET trial: clinical activity and safety of dostarlimab for recurrent or advanced dMMR endometrial cancer patients - Oncology Central
Immune-Related End Points Consistent With Traditional Measurements of Dostarlimab in Non-Endometrial dMMR Solid Tumors
From New Molecular Insights to New Treatment Options in Endometrial Cancer | Published in healthbook TIMES Oncology Hematology
The Time Course of Adverse Events During Dostarlimab Treatment in Patients with Recurrent or Advanced Endometrial Cancer in the

Dostarlimab Shows Durable Antitumor Activity in GARNET Trial Expansion Cohorts With Advanced, Recurrent Endometrial Cancer

Clinical activity and safety of the anti-PD-1 monoclonal antibody dostarlimab for patients with recurrent or advanced dMMR endometrial cancer - Plain Language Summaries

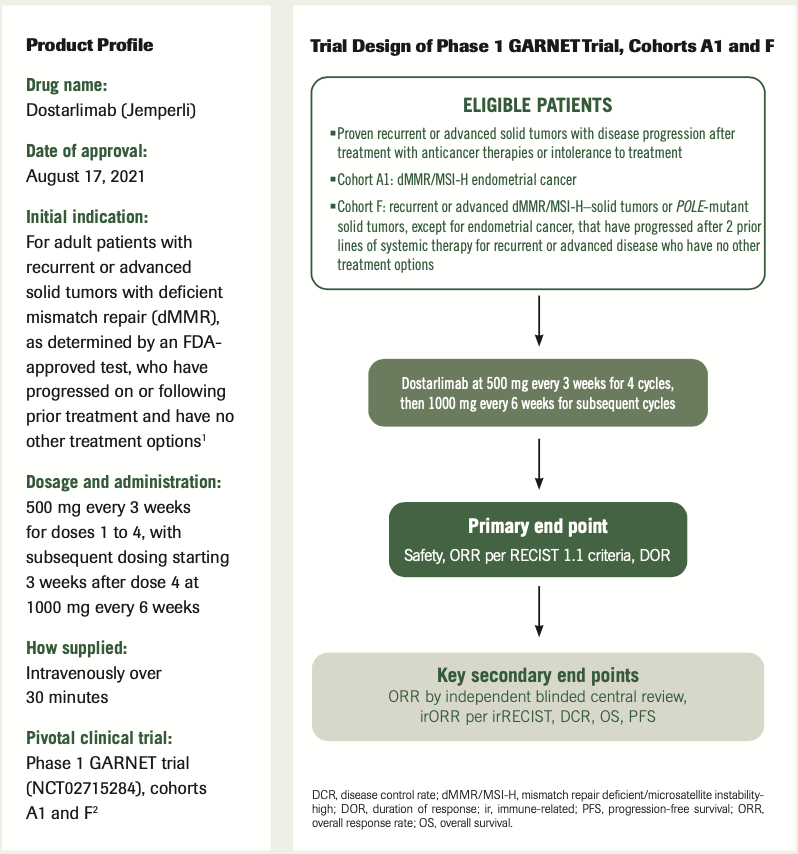
Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET—a phase I ...

Interim analysis of the immune-related endpoints of the mismatch repair deficient (dMMR) and proficient (MMRp) endometrial cancer cohorts from the GARNET study - ScienceDirect

ESMO 2022: Efficacy of dostarlimab in endometrial cancer (EC) by molecular subtype: A post hoc analysis of the GARNET study

272 Dostarlimab in advanced/recurrent mismatch repair deficient/microsatellite instability high or proficient/stable endometrial cancer: the GARNET study | International Journal of Gynecologic Cancer