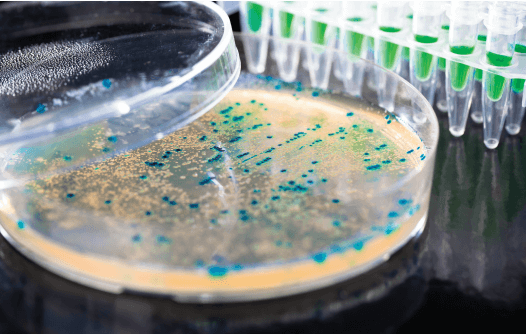
Current technologies to endotoxin detection and removal for biopharmaceutical purification - Schneier - 2020 - Biotechnology and Bioengineering - Wiley Online Library

Occurrence and fate of endotoxin activity at drinking water purification plants and healthcare facilities in Japan - ScienceDirect

USP ; An Evolving Series of Informational Chapters on Depyrogenation | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

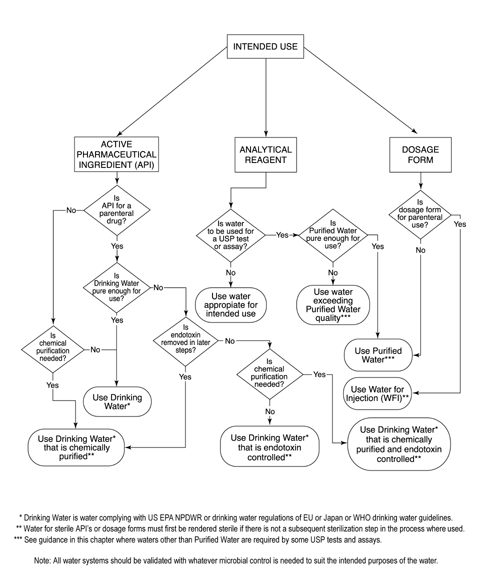
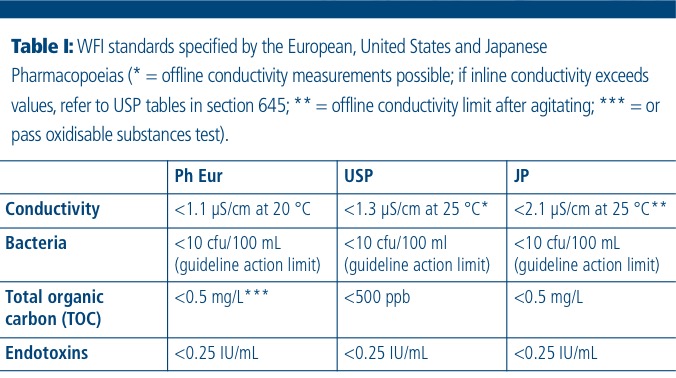
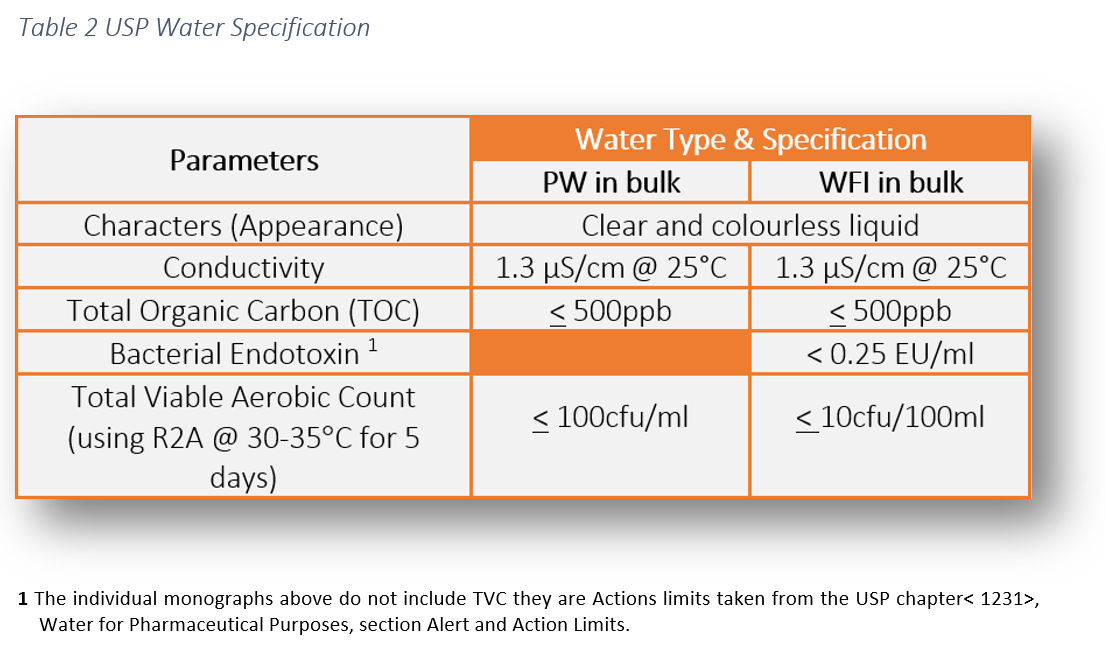
The Design, Control, Monitoring and Validation of Water Systems for Pharmaceuticals, Biologics, Medical Devices, Cosmetics, and Personal Care Products

Will A Proposed Reduction in Endotoxin Limits for Drugs and Biologics Improve Patient Safety? | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology