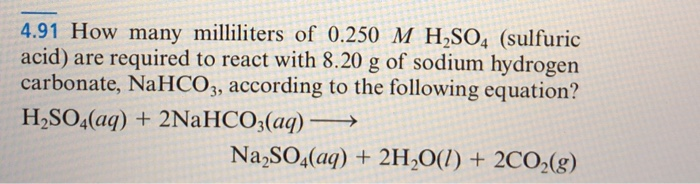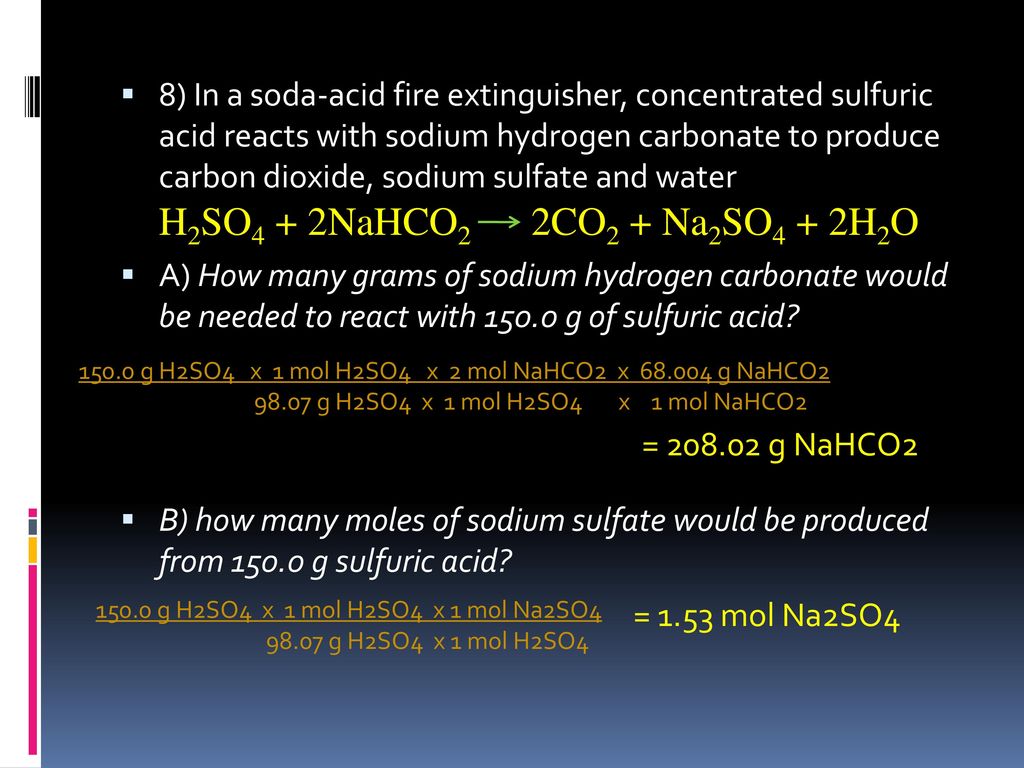Question Video: Identifying the Volatile Compound in a Reaction for Volatilization Gravimetry | Nagwa
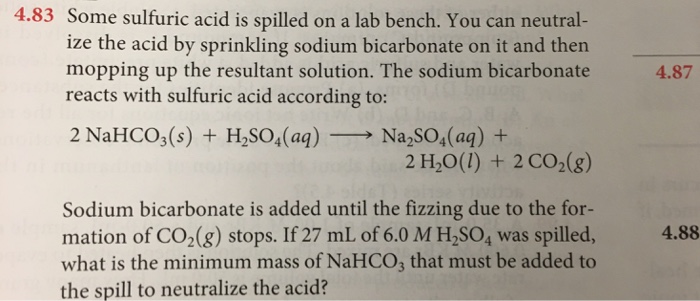
Why do we use sodium carbonate and sulphuric acid in fire extinguisher even though when sodium bicarbonate reacts with sulphuric acid gives the same products? - Quora

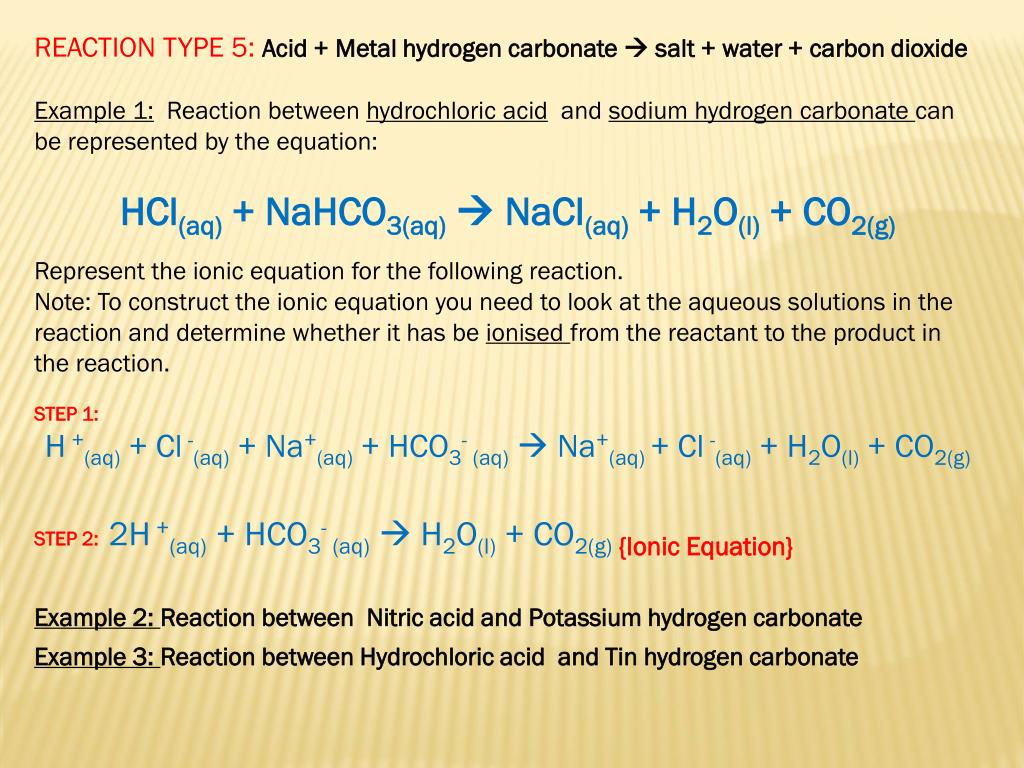
What are Acids? An acid is any compound that yields hydrogen ions (H + ) or hydronium ions (H 3 O + ) when dissolved in water. Hydronium ions are really. - ppt download

The graph of moles of sodium hydrogen carbonate versus moles of sulfuric acid produce a linear graph with the equation y = 2x - 2E-17. The molar mass of sodium hydrogen carbonate
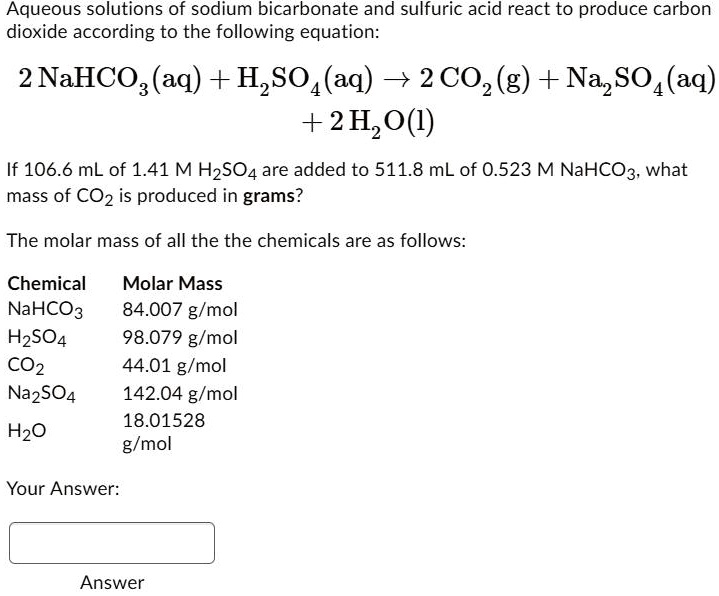
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCO3(aq) + H,SO4(aq) = 2 C02(g) + Na2 SO4(aq) +2H20(1) If 106.6

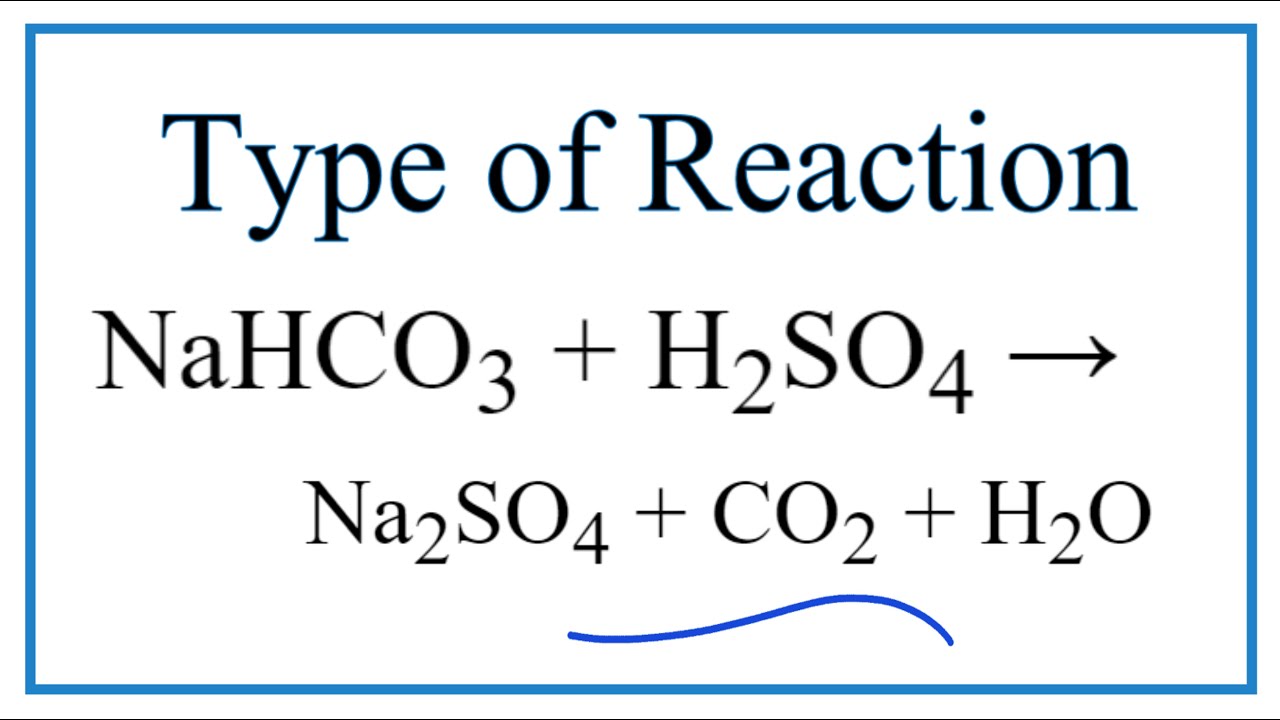
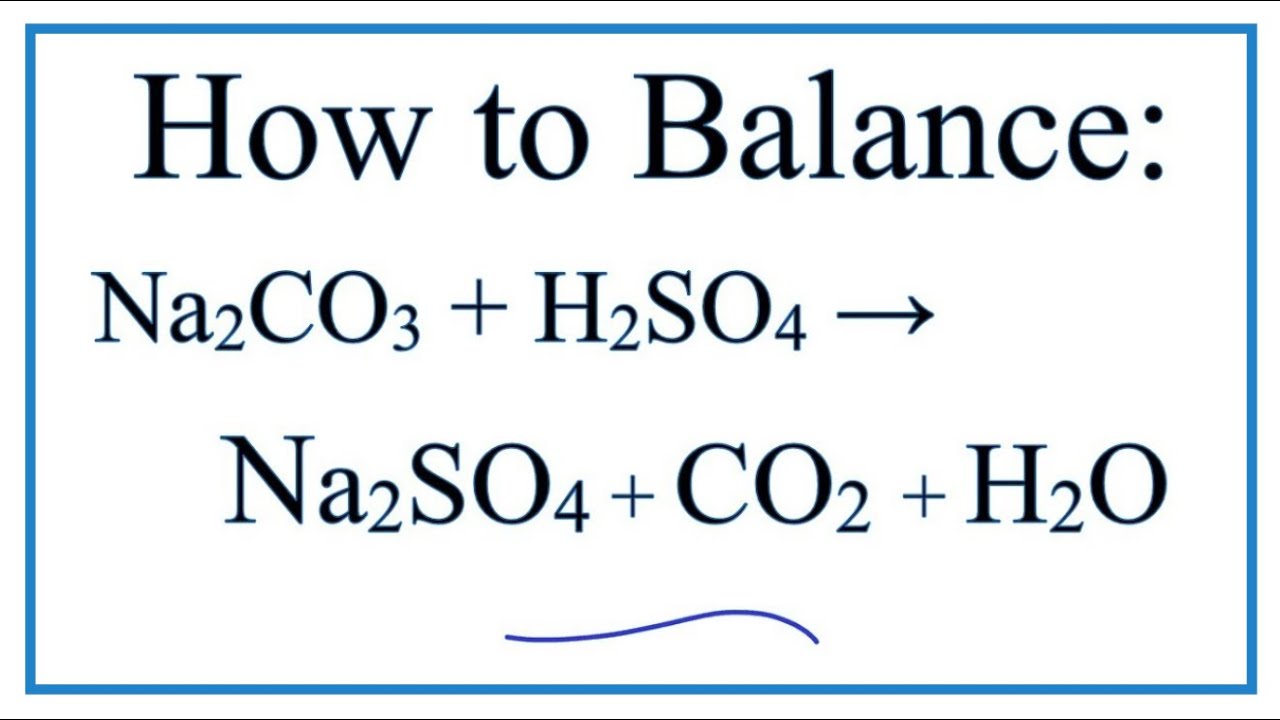
balance the equation Sodium bicarbonate + Sulphuric acid = Sodium sulphate + Water + Carbon dioxide. - Brainly.in
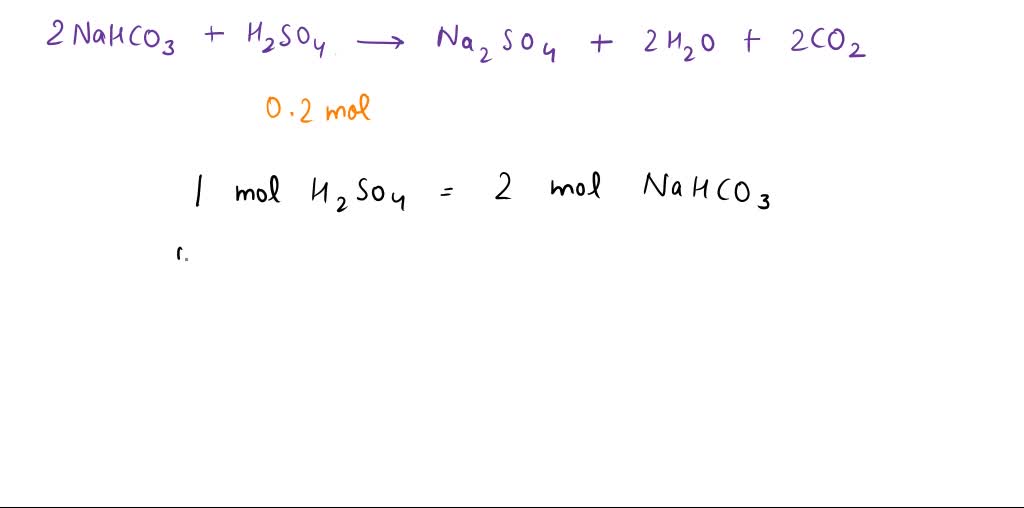
SOLVED: Sodium hydrogen carbonate (NaHCO3) reacts with sulfuric acid (H2SO4) to form sodium sulfate, carbon dioxide and water. What is the mass of sodium hydrogen carbonate required to neutralize 0.200 moles of
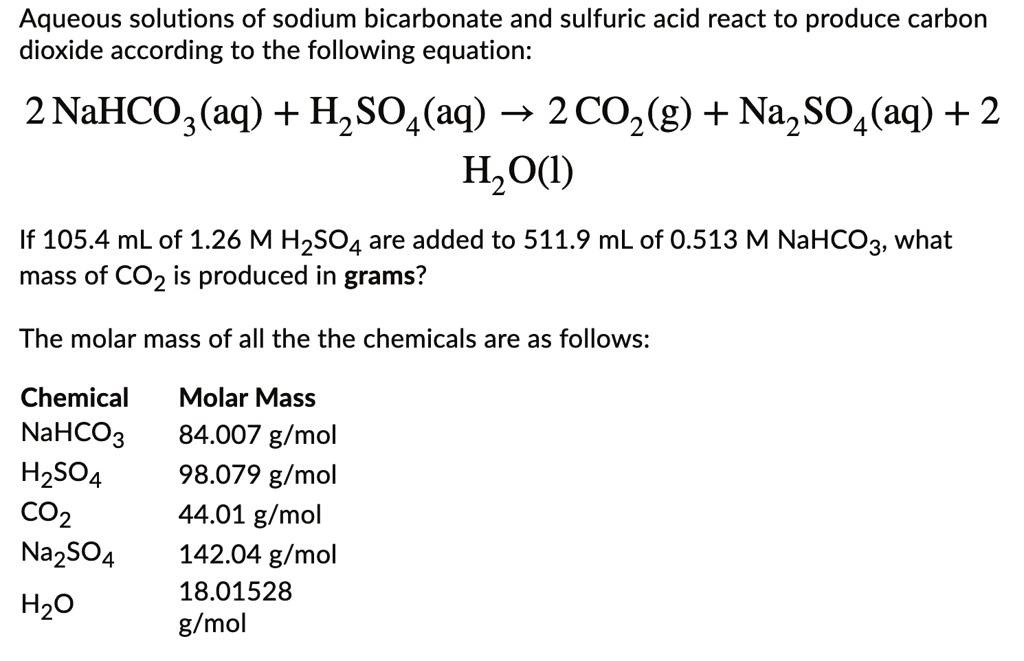
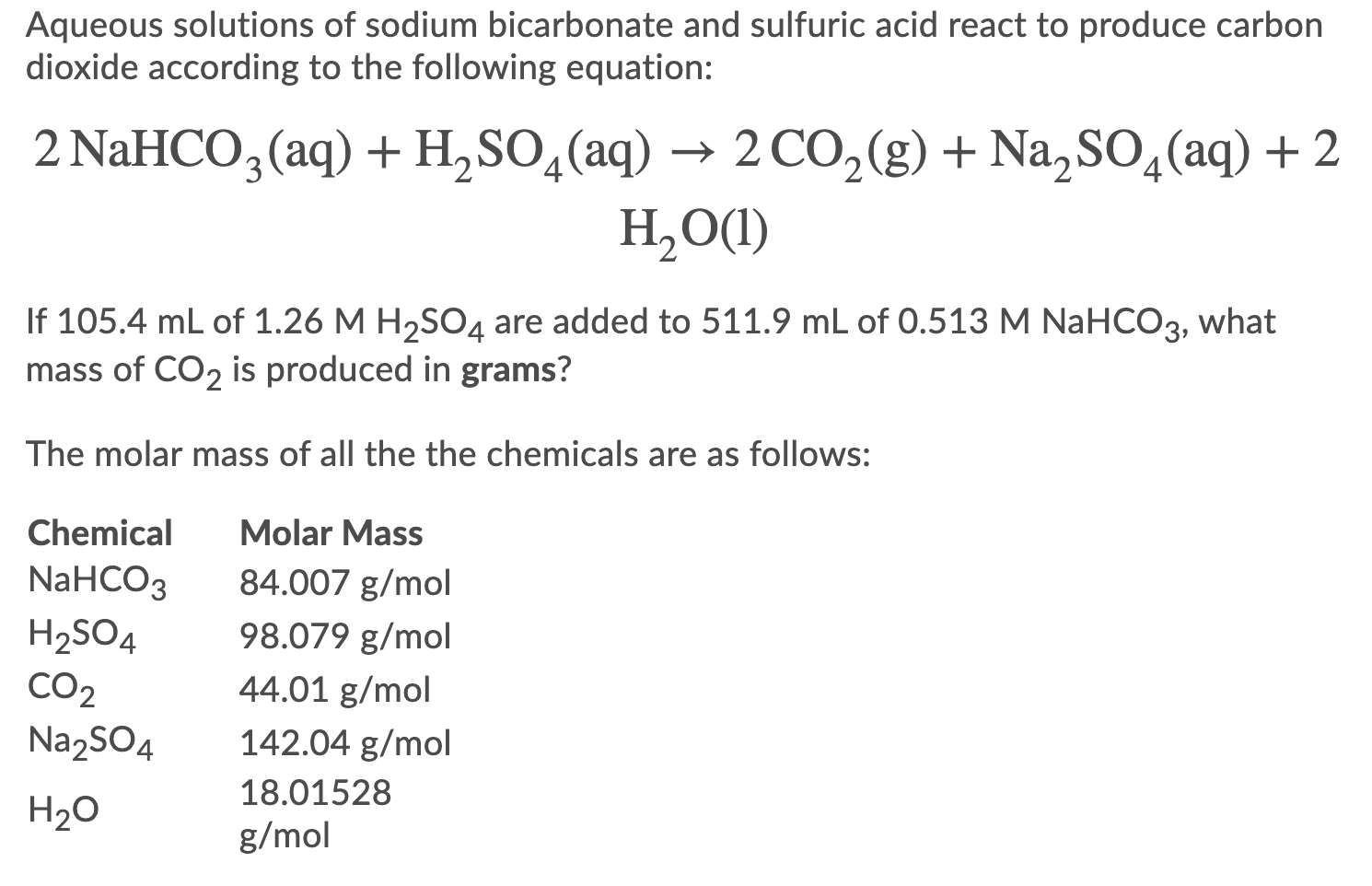
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCOs(aq) + H,SO4(aq) 3 2CO2(g) + NazSO4(aq) + 2 HzO() If 105.4